Peroxide, any of a class of chemical compounds in which two oxygen atoms are linked together by a single covalent bond Several organic and inorganic peroxides are useful as bleaching agents, as initiators of polymerization reactions, and in the preparation of hydrogen peroxide (qv) and other oxygen compoundsOther articles where Barium peroxide is discussed barium Compounds The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengthsStructure and Function Peroxidases are a group of enzymes that catalyze the oxidation of a substrate by hydrogen peroxide or an organic peroxide Most peroxidases are ferric heme proteins;

Hydrogen Peroxide Structure H2o2 Over 100 Million Chemical Compounds Mol Instincts
Peroxide ion structure
Peroxide ion structure-Quantofix ® Peroxide Test Strips (Product Nos Z and Z) In the presence of hydrogen peroxide the test paper turns blue Quantofix ® Peroxide test sticks can also be used for the determination of peracetic acid and other organic and inorganic hydroperoxides To test for hydroperoxides in organic solvents, the test zone is wettedLewis structure of Hydrogen peroxide (H 2 O 2) contains two OH bonds and one OO bond Also, there are two lone pairs on each oxygen atom Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2 Each step of drawing lewis structure of H 2 O 2 is explained in detail in this tutorial


Diacetyl Peroxide C4h6o4 Pubchem
Mar 15, · Warnings Benzoyl peroxide can cause a rare but serious allergic reaction or severe skin irritation Stop using benzoyl peroxide topical and get emergency medical help if you have hives, itching;O 22 (peroxide ion) anion contains only two oxygen atoms Peroxide anion has 2 charge In O 22 lewis structure, each oxygen atom has 1 charge and three lone pairs Both oxygen atoms are joint through a single bondJun 17, 19 · Hydrogen peroxide is commonly used to lighten hair It may be used on its own or in other blonde dyes Hair dye with hydrogen peroxide is considered permanent dye, which means that it will only go
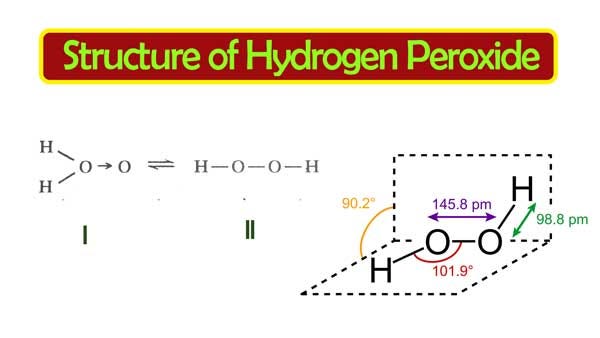
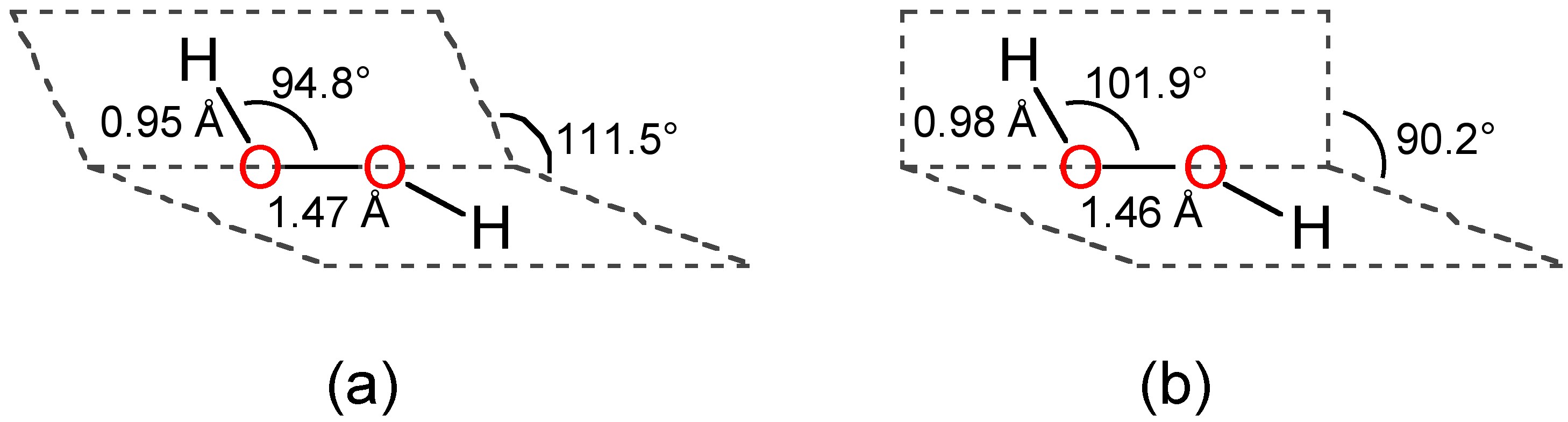
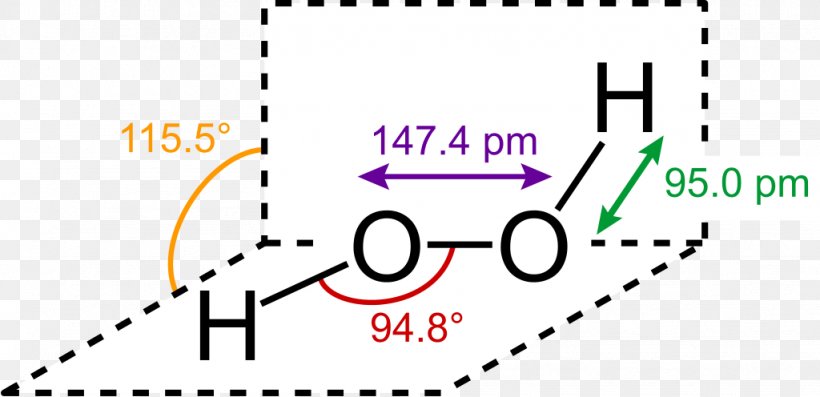
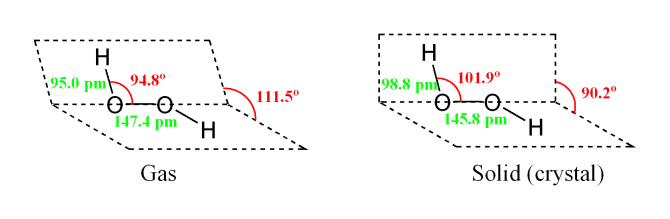
Difficult breathing, feeling lightheaded;The quaternary structure is notable for subunits that bind tightly to one another through " arm exchange " of up to 25 overlapping residues per subunit This interweaving is considered important due to the high reactivity of the hydrogen peroxide substrate (Goodsell, 04;May 03, 19 · The structure of hydrogen peroxide is nonplanar H 2 O 2 has an open book structure with O – O spins The dihedral angle is 111° The OO bond length is 1458 pm and the OH bond length is 9 pm (which is equal to 9 × 10 13 m)
A peroxide is any compound which has two oxygen atoms bonded together The OO group is the peroxide group of the compound And Hydrogen Peroxide is the simplest peroxide The chemical formula for hydrogen peroxide is H2O2 It is a water molecule with one extra atom of oxygen, It has various uses ranging from disinfectant to propellant forApr 01, 1944 · Structure of the Nitrogen Peroxide Molecule LonguetHiggins, H C Abstract HITHERTO three structures for the nitrogen peroxide (N 2 O 4) molecule have received support Publication Nature Pub Date April 1944 DOI /a0 Bibcode 1944NaturLPeroxide cure The final properties of the formed network will depend on the reactivity and structure of the coagent Understanding these structureproperty relationships will allow for more informed coagent selection The present article will review the use of peroxides to cure elastomer systems, and introduce the



Benzoyl Peroxide Acne Treatment Drug Chemical Structure Also Used To Dye Hair And Whiten Teeth Bleaching Conventional Canstock


Methyl Peroxide C2h6o2 Chemspider
The electron geometry of H2O2 is tetrahedral because each oxygen in the Hydrogen peroxide lewis structure has Sp³ hybrid which adopts a tetrahedral structure Whereas molecular geometry of H2O2 is bent because the presence of lone pair on oxygen causes the O–H bond in the H2O2 lewis structure to be pushed downward and upward directionsStructure, properties, spectra, suppliers and links for Hydrogen peroxide, , , , HOOHNov 04, 19 · A peroxide is defined as a polyatomic anion with molecular formula O 22 The compounds are generally classed as ionic or covalent or an organic or inorganic The OO group is termed the peroxo group or peroxide group Peroxide


Hydrogen Peroxide How To Make Hydrogen Peroxide Uses Properties By Chemistry Page Medium


Cyclohexanone Peroxide 78 18 2
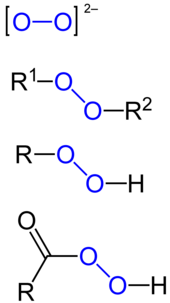
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste Small amounts of gaseous hydrogen peroxide occur naturally in the air Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat Although nonflammable, it is a powerful oxidizing agent that can cause spontaneous combustion when it comes in contact with organic materialHydrogen peroxideChemical compound Methyl ethyl ketone peroxide (MEKP) is an organic peroxide, a high explosive similar to acetone peroxide MEKP is a colorless, oily liquid whereas acetone peroxide is a white powder at STP;Jan 08, 18 · Main Difference – Peroxide vs Superoxide An oxide is any chemical compound that contains one or more oxygen atoms Oxides can be oxides containing oxide anions (O 2), peroxides containing peroxide anions (O –) or superoxides containing superoxide anion (O 2 –)A peroxide is any compound that is composed of an oxygenoxygen single bondThis can be either in the form


Hydrogen Peroxide Molecule Of The Month September 06 Html Version


Hydrogen Peroxide Structure Line Icon Stock Vector Illustration Of Outline Bleach
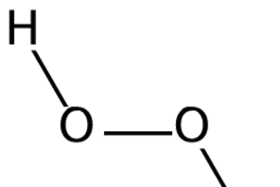
Carbamide peroxide bleaching agents have raised important questions on their potential adverse effects on the structure of enamel The purpose of this study was to examine the effects of three carbamide peroxide bleaching agents in different concentrations (10, 16 and 35%) on the structureStructure Find Similar Structures Molecular Formula C2H6O2 Synonyms Dimethyl peroxide Peroxide, dimethyl Methyl peroxide (Methylperoxy)methaneThe Benzoyl Peroxide is an oxidizer, which is generally utilised in the generation of polymers Structure of Benzoyl Peroxide The chemical structure of a molecule in the Benzoyl Peroxide involves the arrangement of the atoms and the chemical bonds that holds all the atoms collectively The Benzoyl Peroxide molecule comprises 29 bonds


Hydrogen Peroxide H2o2 Molecular Structure Isolated On Black Stock Illustration Illustration Of People Chemical


Sodium Carbonate Hydrogen Peroxide 2 1 1 Ch2na2o5 Chemspider
One notable exception being the glutathione peroxidase, which is a seleniumcontaining enzyme They are present in virtually all living speciesA stepbystep explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide) Note that the H2O2 Lewis structure is frequently used on tests aA superoxide is a compound that contains the superoxide ion, which has the chemical formula O − 2 The systematic name of the anion is dioxide(1−)The reactive oxygen ion superoxide is particularly important as the product of the oneelectron reduction of dioxygen O 2, which occurs widely in nature Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons,


Is Benzoyl Peroxide The Same As Hydrogen Peroxide Quora



Hydrogen Peroxide Structure H2o2 Over 100 Million Chemical Compounds Mol Instincts
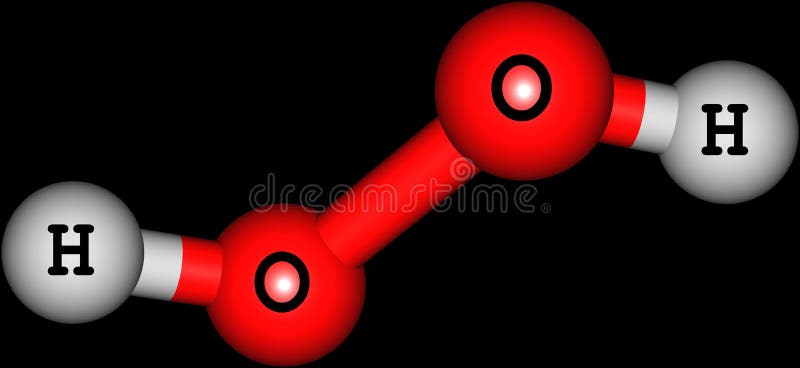
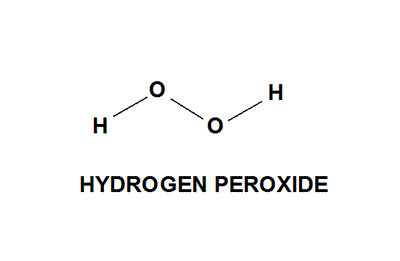
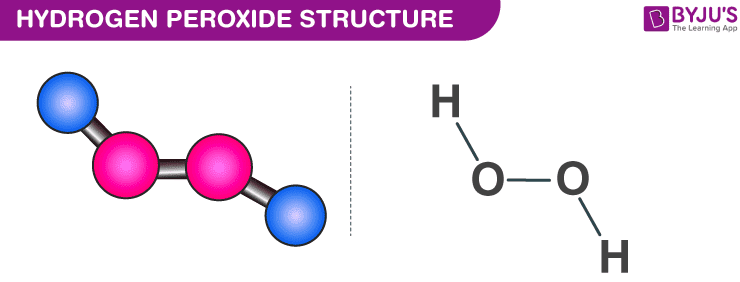
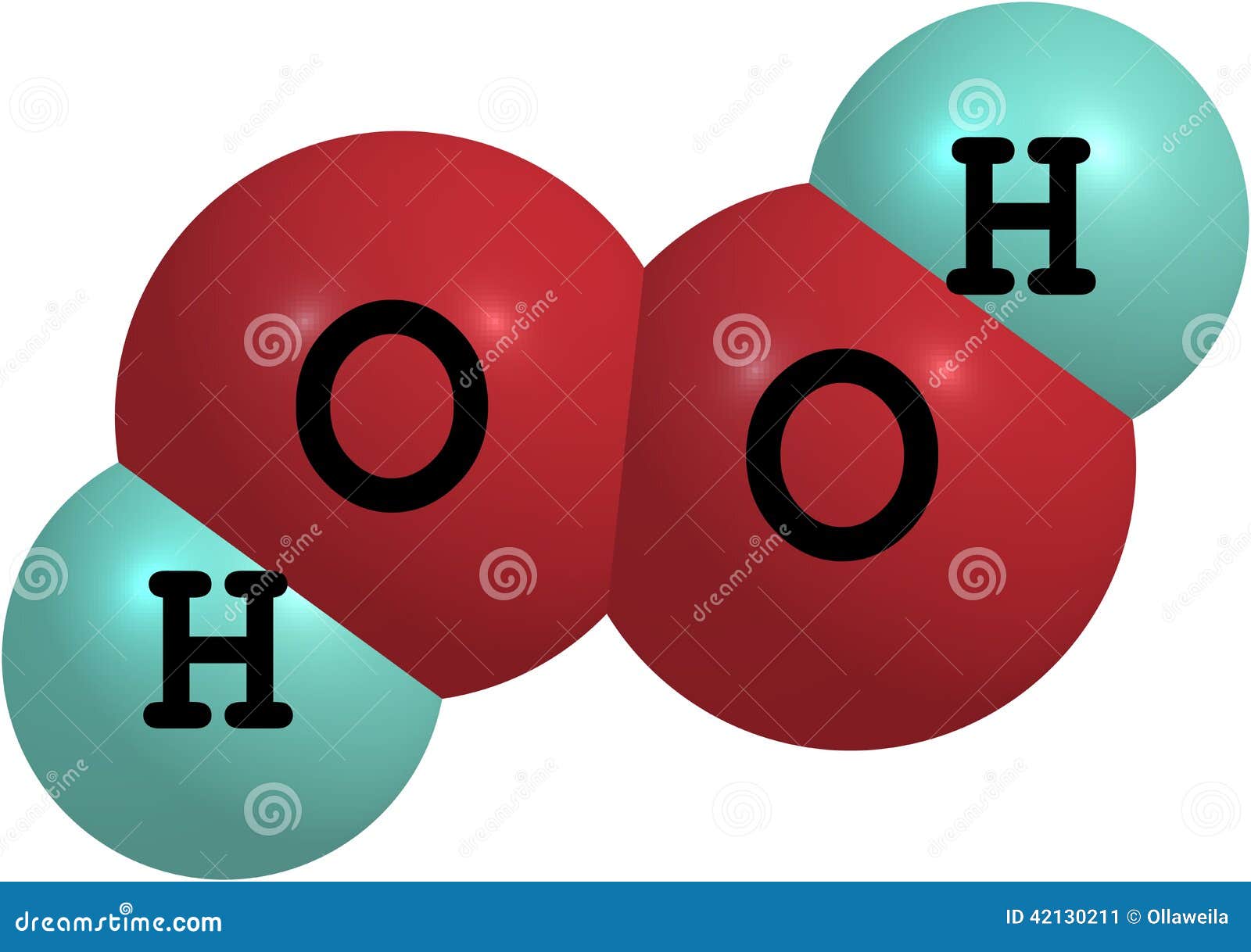
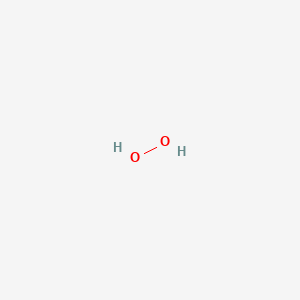
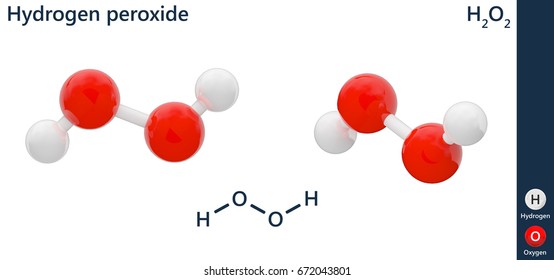
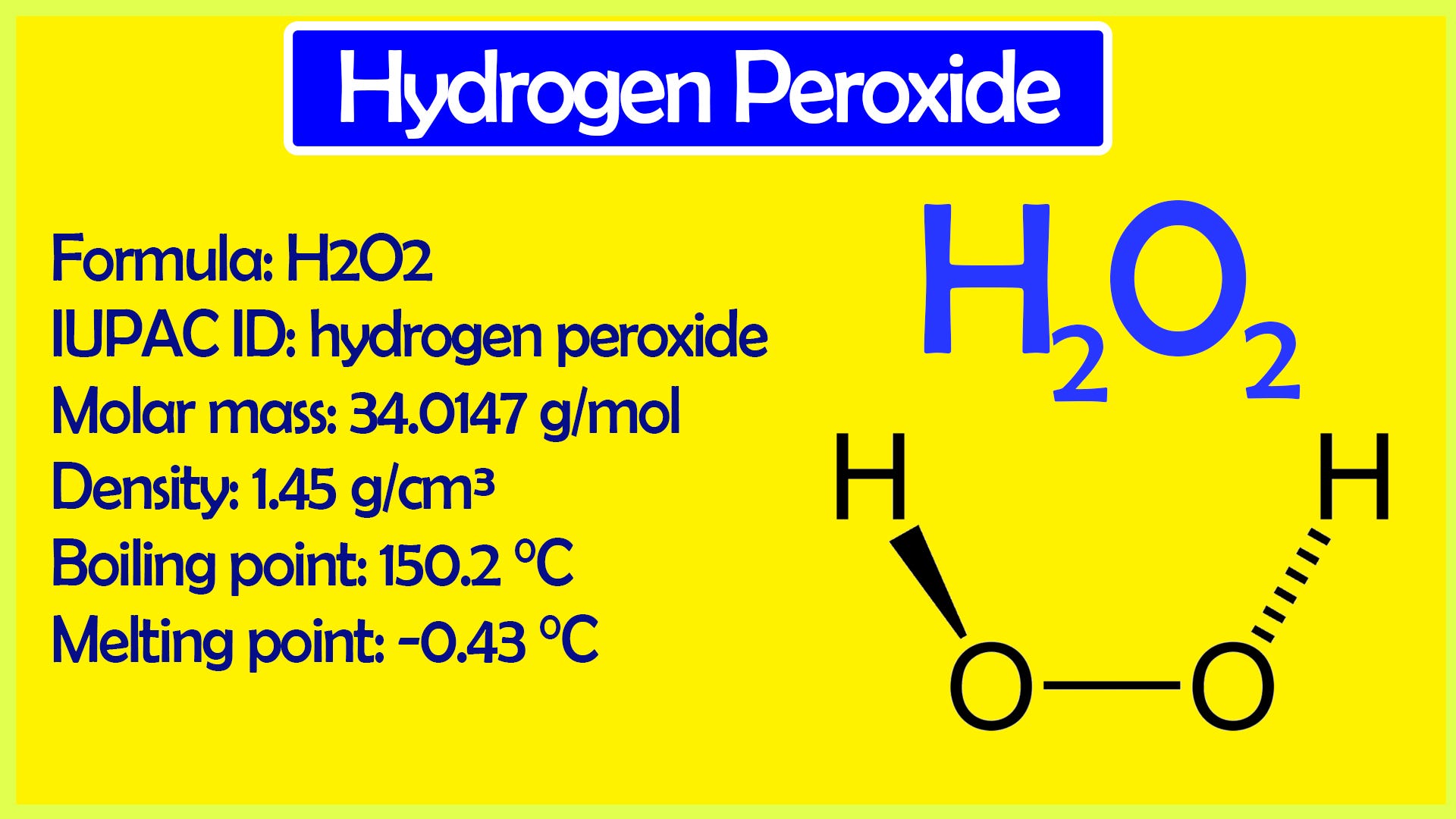
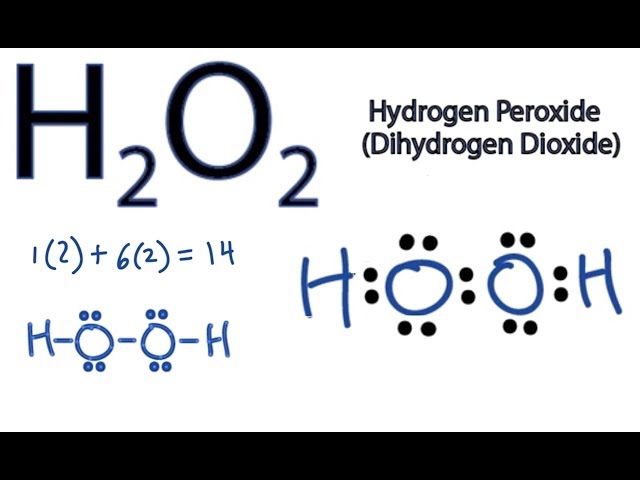
In gaseous state hydrogen bonding is absent where is inHydrogen Peroxide Structure Oxygen and Water Posted on October 18, 09 by admin Hydrogen peroxide is a naturally occuring compound formed within the cell structures of plants and animals, in the earth's atmosphere and in the waters that cover the earthThe form of hydrogen peroxide H2O2 is H—O—O—H, with each dash reflecting a single covalent bond That is, each bond contains a pair of mutual electrons, formally one from each of the atoms at the bond's ends The H—O bonds are polar, while the O—O bonds are not



Hydrogen Peroxide 30 Baker Analyzed Acs J T Baker 1 L Hydrogen Peroxide 30 Baker Analyzed Acs J T Baker Fisher Scientific


Tdxdn W8tidhxm
Structure of peroxide & structure of peroxide online Wholesalers choose structure of peroxide from 33 list of China structure of peroxide ManufacturersMEKP is slightly less sensitive to shock and temperature, and more stable in storage Depending on the experimental conditions, several different adducts of methyl ethyl ketone and hydrogen peroxideStructure, properties, spectra, suppliers and links for Isopropyl peroxide
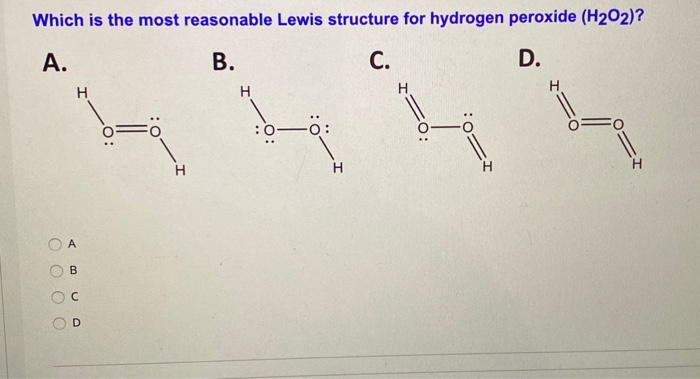


Solved Which Is The Most Reasonable Lewis Structure For H Chegg Com
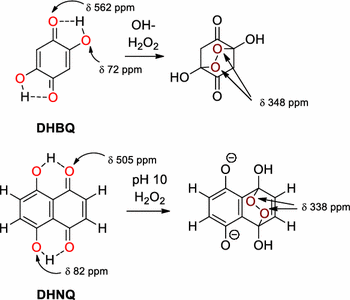


Cyclic Peroxides As Key Intermediates In The Degradation Of Cellulosic Key Chromophores By Alkaline Hydrogen Peroxide First Direct Proof By 17 O Nmr Springerlink
H2O2, hydrogen peroxide is a funny looking molecule It has two oxygen atoms in the centre, and they end up singlebonded Then, there is a Hydrogen Atom atMSLRPWGRFCKNIZUHFFFAOYSAJ Sodium carbonate peroxide Similar structures search, synonyms, formulas, resource links, and other chemical informationBis(2,4dichlorobenzoyl) peroxide C14H6Cl4O4 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more


Chromium Vi Oxide Peroxide Chemistry Lewis Structure Png 1024x918px Chromiumvi Oxide Peroxide Area Black And White
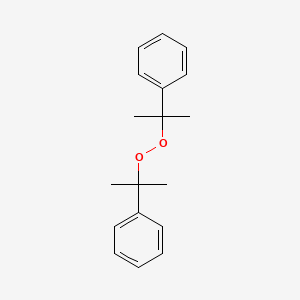


Dicumyl Peroxide C18h22o2 Pubchem
First recognized as a chemical compound in 1818, hydrogen peroxide is the simplest member of the class of peroxidesOf the several processes of manufacture, the principal ones involve reactions of oxygen from the air with certain organic compounds, especially anthraquinone or isopropyl alcoholMajor commercial grades are aqueous solutions containing 35, 50, 70, or 90 percent hydrogen peroxideWe probed the effect of 3 commonly used oxidants, hydrogen peroxide, tertbutyl hydroperoxide, and 2,2'Azobis(2amidinopropane) dihydrochloride (AAPH), on a therapeutic monoclonal IgG1 antibody (mAb8) Upon oxidation, mAb8 did not show noticeable changes in its secondary structure but showed minor changes in tertiary structureOf organic and inorganic peroxide molecules Essentially all of the features of peroxide reactivity are associated with the tendency for spontaneous change to form more stable products The unusual weakness of theOO bond is probably a consequence of the molecular and electronic structure of peroxide molecules and of the relatively high



Hydrogen Peroxide Formula H2o2 Over 100 Million Chemical Compounds Mol Instincts
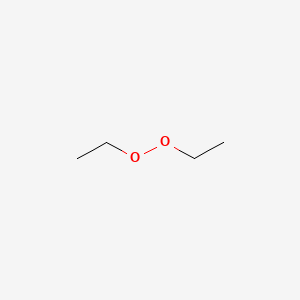


Diethyl Peroxide C4h10o2 Pubchem
Benzoyl Peroxide is a peroxide with antibacterial, irritant, keratolytic, comedolytic, and antiinflammatory activity Upon topical application, benzoyl peroxide decomposes to release oxygen which is lethal to the bacteria Proprionibacterium acnes Due to its irritant effect, benzoyl peroxide increases turnover rate of epithelial cells, thereby peeling the skin and promoting the resolutionPeroxides are a group of compounds with the structure R−O−O−R, where R = any element The O−O group in a peroxide is called the peroxide group or peroxo group The nomenclature is somewhat variable The most common peroxide is hydrogen peroxide (H 2 O 2), colloquially known simply as "peroxide" It is marketed as solutions in water at various concentrationsLauroyl peroxide C24H46O4 CID 7773 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more COVID19 Information Public health information (CDC) Research information (NIH) SARSCoV2 data (NCBI)


Hydrogen Peroxide H2o2 Molecule Stock Illustration Illustration Of Compound Atom
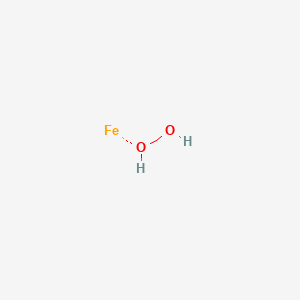


Hydrogen Peroxide Iron Feh2o2 Pubchem
The dimer is known as diacetone diperoxide (DADP) Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach like odor (when impure) or a fruitlike smell when pure, and can explode powerfully if subjected to heat, friction, static electricity, concentrated sulfuric acid, strong UV radiation or shockDec 04, 19 · Structure of hydrogen peroxide is nonlinear, nonplanar molecule It has a open book structure The –OO linkage is called peroxy linkage The structure of H 2 O 2 is significantly different in gaseous state and crystalline solid state The difference is because of hydrogen bonding;OxyR, a bacterial peroxide sensor, is a LysRtype transcriptional regulator (LTTR) that regulates the transcription of defense genes in response to a low level of cellular H2O2 Consisting of an Nterminal DNAbinding domain (DBD) and a Cterminal


Hydrogen Peroxide Chemical Structure Ozident


Carbamide Peroxide Ch6n2o3 Pubchem
Hydrogen peroxide (H 2 O 2) was discovered in 1818 and has been commercially available since the nineteenth century Hydrogen peroxide is one of the most common bleaching agents The positive aspects of hydrogen peroxide include the fact that it is highly environmentfriendly (decomposes to O 2 and H 2 O), colourless and noncorrosive It is aMay 06, 19 · Hydrogen Peroxide Structure, Uses and Properties Hydrogen peroxide is a highly unstable chemical compound Two molecules of hydrogen combine with two molecules of oxygen to form hydrogen peroxide Hence, its chemical formula is H 2 O 2Initiation By Diacyl Peroxides Free radicals can be generated by a number of compounds, called initiators 1 One of the most important classes of initiators are diacyl peroxides, which have the general formula R 1 C(O)OOC(O)R 2, wherein R 1 and R 2 represent alkyl and/or aryl groups All diacyl peroxides are thermally unstable and decompose at relative low temperatures thereby


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses


Hydrogen Peroxide Soundbite Rsc Education



Tert Butyl Peroxide Cas 110 05 4 Scbt Santa Cruz Biotechnology
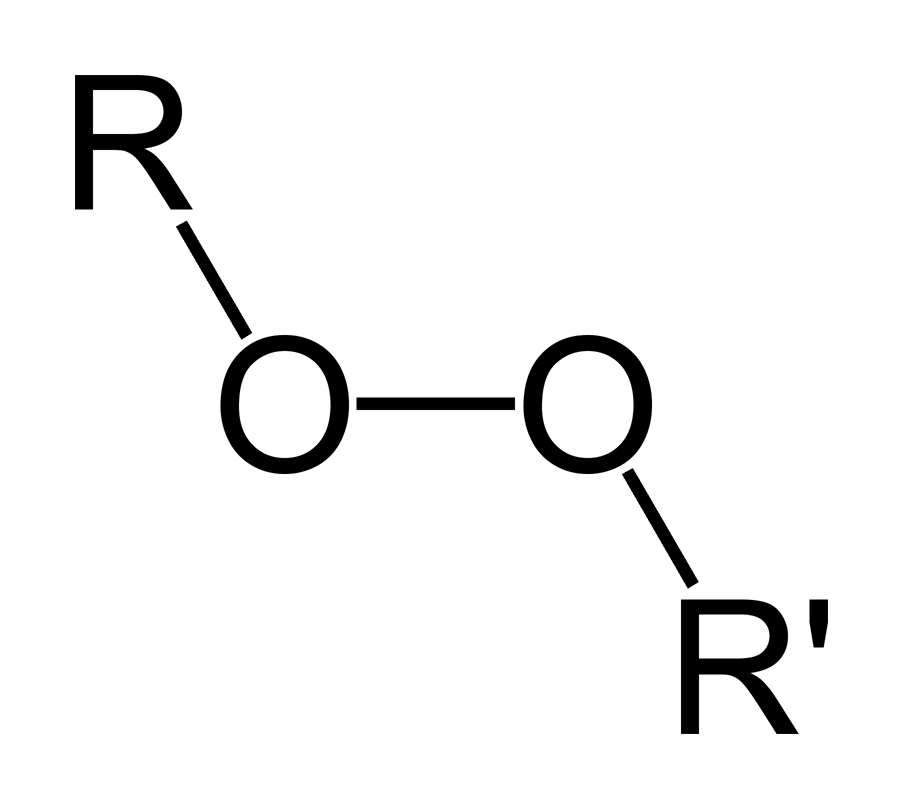


Organic Peroxide Wikipedia



Effect Of Peroxide Versus Alkoxyl Induced Chemical Oxidation On The Structure Stability Aggregation And Function Of A Therapeutic Monoclonal Antibody Journal Of Pharmaceutical Sciences


Hydrogen Peroxide Structure Oxygen And Water



The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Molecules Tech Company Logos
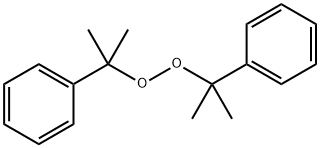


Dicumyl Peroxide Cas 80 43 3
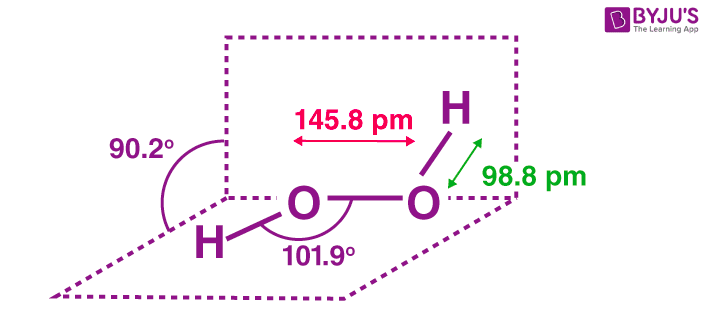


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses


Sodium Peroxide Na2o2 Chemspider



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Stock Photo Picture And Royalty Free Image Image



The Equilibrium Structure Of Hydrogen Peroxide Sciencedirect
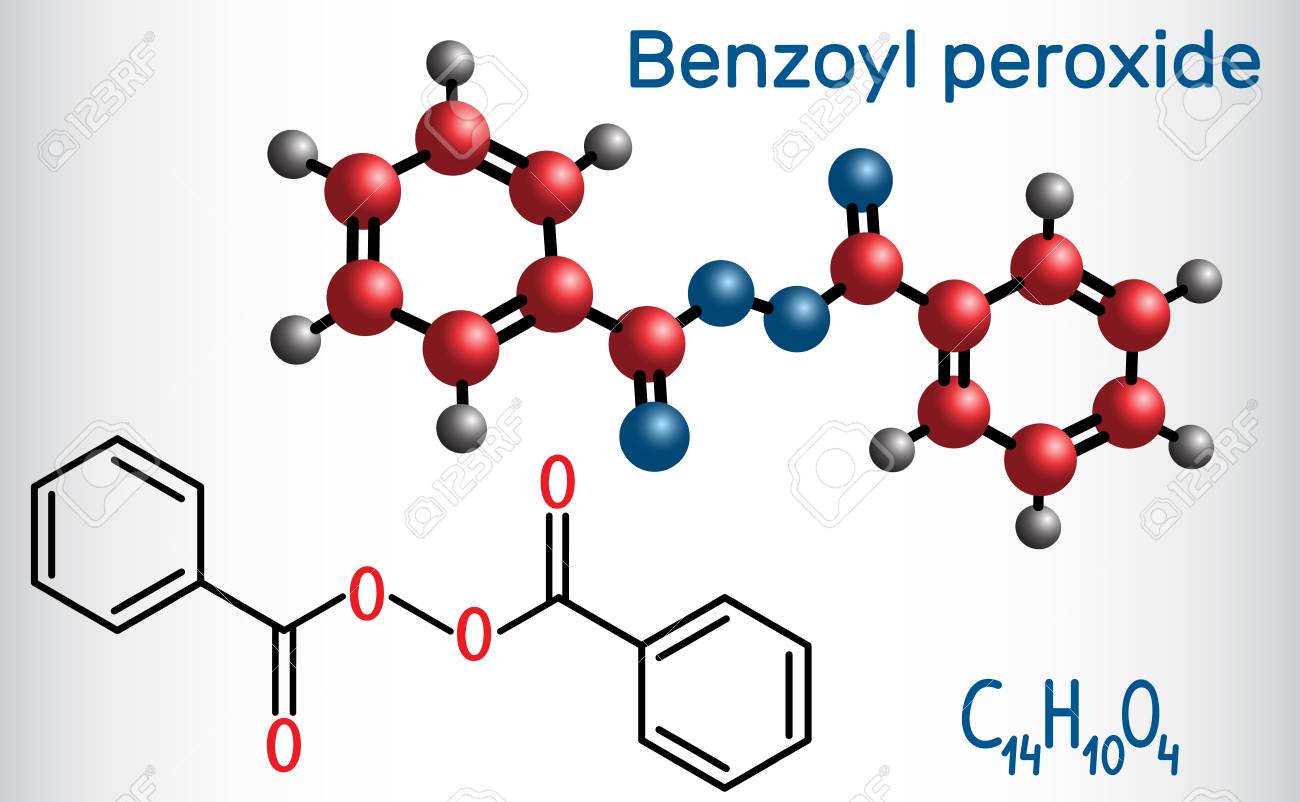


Benzoyl Peroxide Bpo Molecule Structural Chemical Formula Royalty Free Cliparts Vectors And Stock Illustration Image


Baseline Hydrogen Peroxide Seastar Chemicals


Hydrogen Peroxide H2o2 Molecular Structure Isolated On White Stock Illustration Illustration Of Hair Atom


Hydrogen Peroxide H2o2 Pubchem



Hydrogen Peroxide Simple English Wikipedia The Free Encyclopedia


9 4 Hydrogen Peroxide Chemistry Libretexts



Why Does The Extra Oxygen Atom In Hydrogen Peroxide H2o2 Make It An Antiseptic While Water H2o Is Not Antiseptic Quora


H2o2 High Res Stock Images Shutterstock



H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Molecular Geometry Molecular Shapes Intermolecular Force
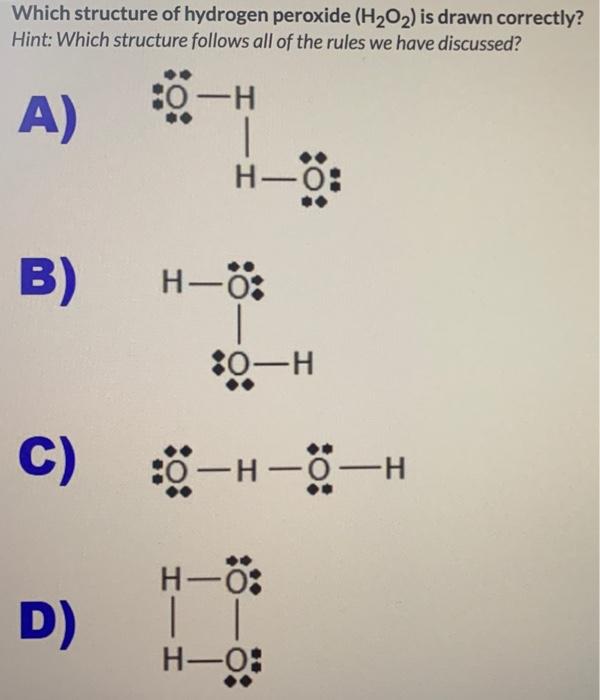


Solved Which Structure Of Hydrogen Peroxide H2o2 Is Dra Chegg Com



Peroxide Wikipedia



Hydrogen Peroxide Molecule Chemical Compound Lewis Structure Decomposition Hydrogen Catalysis Png Pngegg



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube



Hydrogen Peroxide Properties And Uses H2o2



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Royalty Free Cliparts Vectors And Stock Illustration Image


Hydrogen Peroxide How To Make Hydrogen Peroxide Uses Properties By Chemistry Page Medium


Magnesium Peroxide Mgo2 Chemspider


Chemidplus 124 43 6 Aqljvwufpcuvlo Uhfffaoysa N Carbamide Peroxide Usp Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information



File H2o2 Urea Complex Code Png Wikipedia


Peroxide Wikipedia



Hydrogen Peroxide Molecular Structure Isolated On White Stock Photo Download Image Now Istock



What Is Meant By Peroxide Quora


Diethyl Ether Peroxide Wikipedia



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Stock Photo Picture And Royalty Free Image Image



Benzoyl Peroxide Wikipedia



Dicumyl Peroxide Wikidata
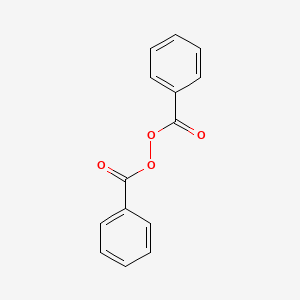


Benzoyl Peroxide C14h10o4 Pubchem



Diacetyl Peroxide C4h6o4 Pubchem


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png 1024x496px Lewis Structure Area Brand Chemical Bond Chemical


Hydrogen Peroxide H2o2 Molecular Structure Isolated On White Stock Illustration Illustration Of Structure People



Chemical Name And Structure Of Benzoyl Peroxide A And Its Impurities Download Scientific Diagram



Part Of The Crystal Structure Of Dabco Bis Perhydrate 1 Showing One Download Scientific Diagram


Peroxide Wikipedia


File H2o2 Structure Png Wikimedia Commons



6 Structure Of Hydrogen Peroxide Drogen Peroxide 2d Png Download Scientific Diagram



Draw The Lewis Structure For Hydrogen Pero Clutch Prep



File H2o2 Solid Structure Svg Wikimedia Commons


Carbamide Peroxide Chemical Structure Molecular Formula Reference Standards


Diethyl Peroxide C4h10o2 Chemspider


Hydrogen Peroxide Definition Formula Structure Properties Uses
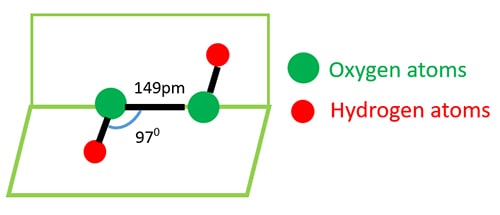


Hydrogen Peroxide Reactions And Physical Properties H2o2



File Mek Peroxide Structure Png Wikimedia Commons
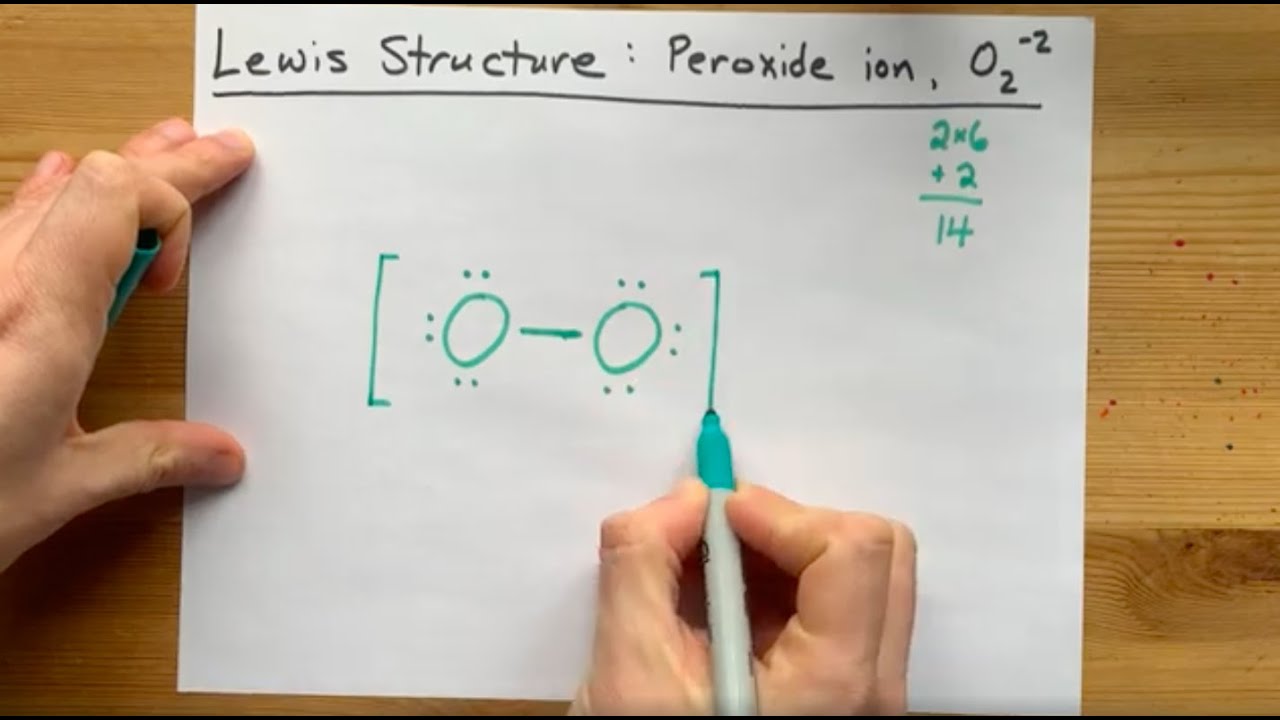


Lewis Structure Of The Peroxide Ion O2 2 Youtube
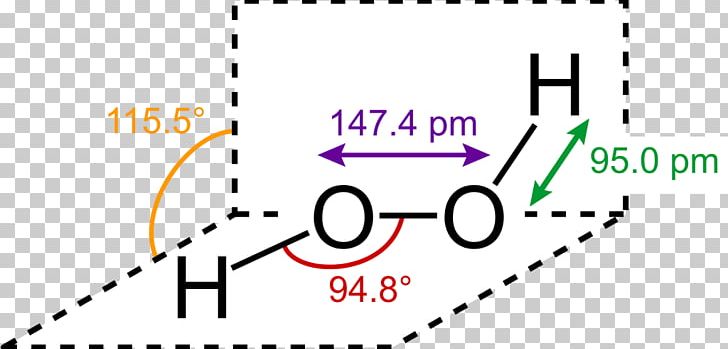


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png Clipart Angle Brand Chemical Bond Chemical Compound Chemical


The Chemical Structure Of A Hydrogen Peroxide B Organic Peroxide Download Scientific Diagram


Illustrated Glossary Of Organic Chemistry Peroxide



Peroxide


Sodium Peroxide Na2o2 Pubchem
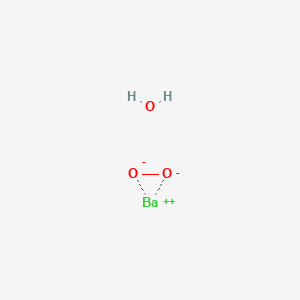


Barium Peroxide Hydrate Bah2o3 Pubchem



Figure 1 From Confirmation Of The Molecular Structure Of Tetramethylene Diperoxide Dicarbamide Tmdd And Its Sensitiveness Properties Semantic Scholar


Hydrogen Peroxide H2o2 Chemspider



Scheme 2 Artemisinin Structure Solid Lines Indicating The Relevant Download Scientific Diagram



Hydrogen Peroxide H2o2 Lewis Structure Novocom Top



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Stock Photo Picture And Royalty Free Image Image


H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Youtube


Structural Chemical Formula And Molecular Structure Of Hydrogen Peroxide H2o2 Chemical Structure Model Ball And Stick 3d Illustration Larastock


File Acetone Peroxide Trimer Structural Formula V 1 Svg Wikimedia Commons


H2o2 High Res Stock Images Shutterstock


Illustrated Glossary Of Organic Chemistry Peroxide
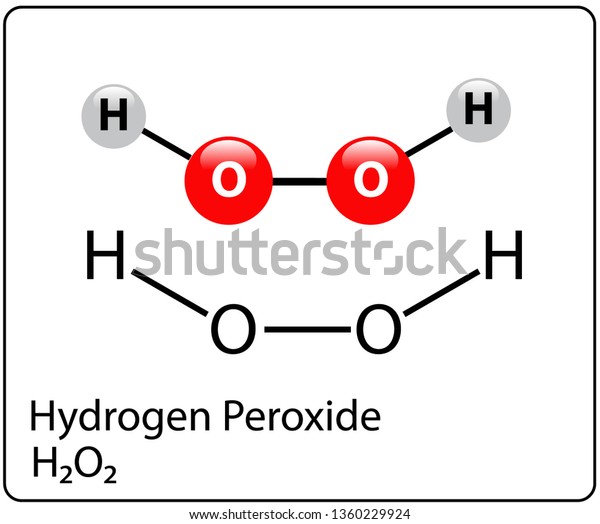


Hydrogen Peroxide Molecule Structure Stock Vector Royalty Free
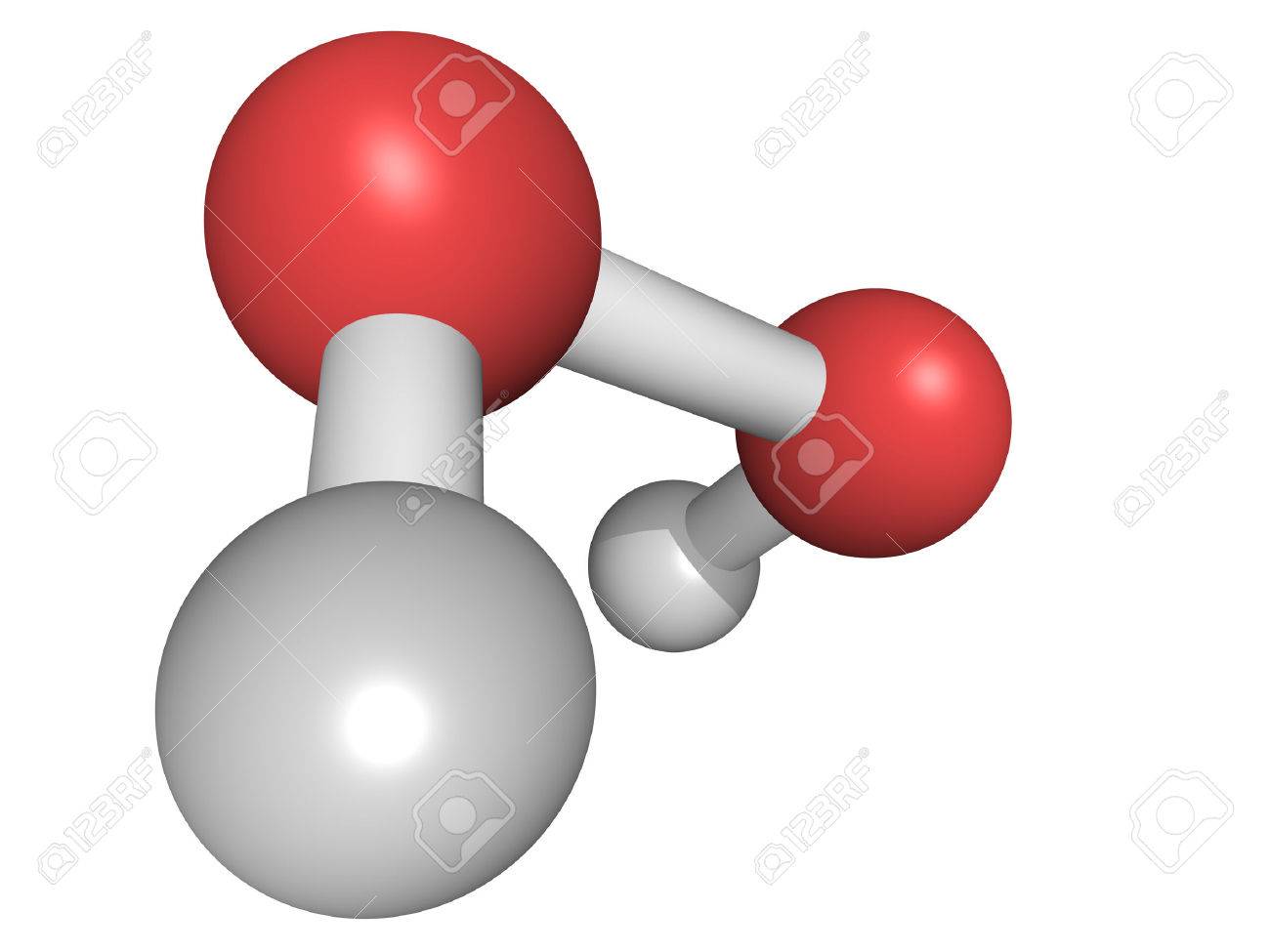


Chemical Structure Of A Hydrogen Peroxide H2o2 Molecule Hooh Stock Photo Picture And Royalty Free Image Image



Food Grade Hydrogen Peroxide Food Grade Hydrogen Peroxide Hydrogen Peroxide Food Grade


Barium Peroxide Bao2 Chemspider


Hydrogen Peroxide Structure Uses And Properties Of Hydrogen Peroxide
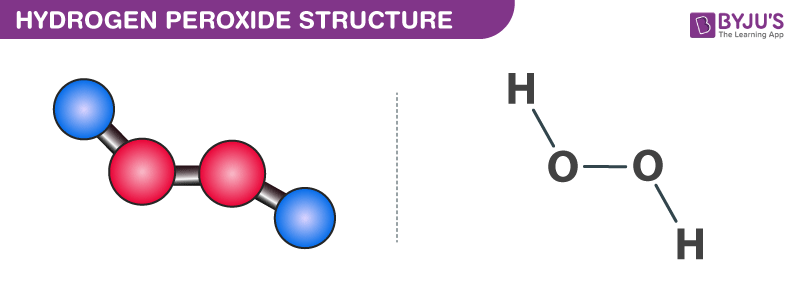


Chemical Makeup Of Hydrogen Peroxide Saubhaya Makeup
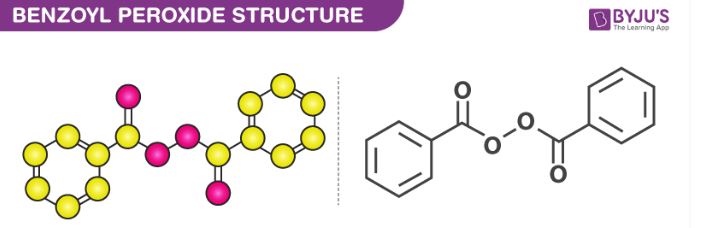


Benzoyl Peroxide Formula Structure Properties And Uses



0 件のコメント:
コメントを投稿